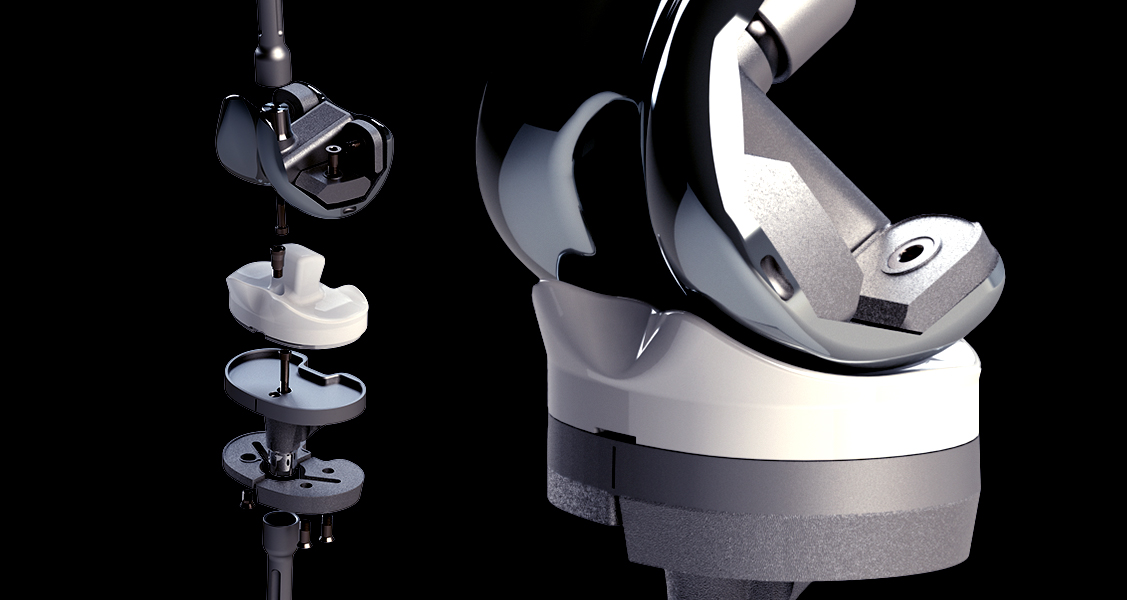
This “First-in-man” study aims to evaluate the clinical performance, effectiveness and safety of this new device. It constitutes an essential step in the process of obtaining CE marking for the device and its instrumentation.
Specifically designed for complex cases of osteoarthritis or prosthetic revisions, this modular device integrates adjustable components, such as wedges and extensions. It thus offers surgeons optimal flexibility, allowing the solution to be precisely adapted to the unique needs of each patient. With this innovation, FH ORTHO confirms its commitment to offering orthopedic solutions at the cutting edge of innovation, adapted to the most demanding situations.
Thanks to these structured initiatives, FH ORTHO applies the highest regulatory and normative standards, in particular those necessary to maintain CE marking, while contributing to the continuous improvement of our medical solutions.